Human Injectable
Nandrolone Decanoate BP 50 mg/ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Nandrolone Decanoate BP 50 mg/ml
Therapeutic Class: Anabolic steroid (Androgens), Hormone in bone formation by stimulation
Indication: Hydeca is an injectable preparation of Nandrolone Decanoate which is an anabolic steroid. Nandrolone Decanoate is indicated in Established Osteoporosis, Disseminated breast cancer in women (palliative therapy), Protein deficiency states occurring after major surgery or trauma, Anemia due to chronic renal failure, Aplastic anemia, Anemia due to cytotoxic therapy, Chronic debilitating disease in elderly, Postsurgical and post traumatic catabolism, During glucocorticosteroid therap.
Dosage & Administration: Established Osteoporosis: 50 mg every 3 weeks Disseminated breast cancer in women (palliative therapy): 50 mg every 3 weeks Protein deficiency states occurring after major surgery or trauma: 50 mg every 2-3 weeks Anemia due to chronic renal failure: 50-200 mg per week Aplastic anemia: 50-150 mg per week Anemia due to cytotoxic therapy: 200 mg per week Chronic debilitating disease in elderly: 100 mg Postsurgical and post-traumatic catabolism: 25-50 mg every 3 weeks During glucocorticosteroid therapy: 50 mg every 2-3 week
Preparation: 1 ml X 1 Injection
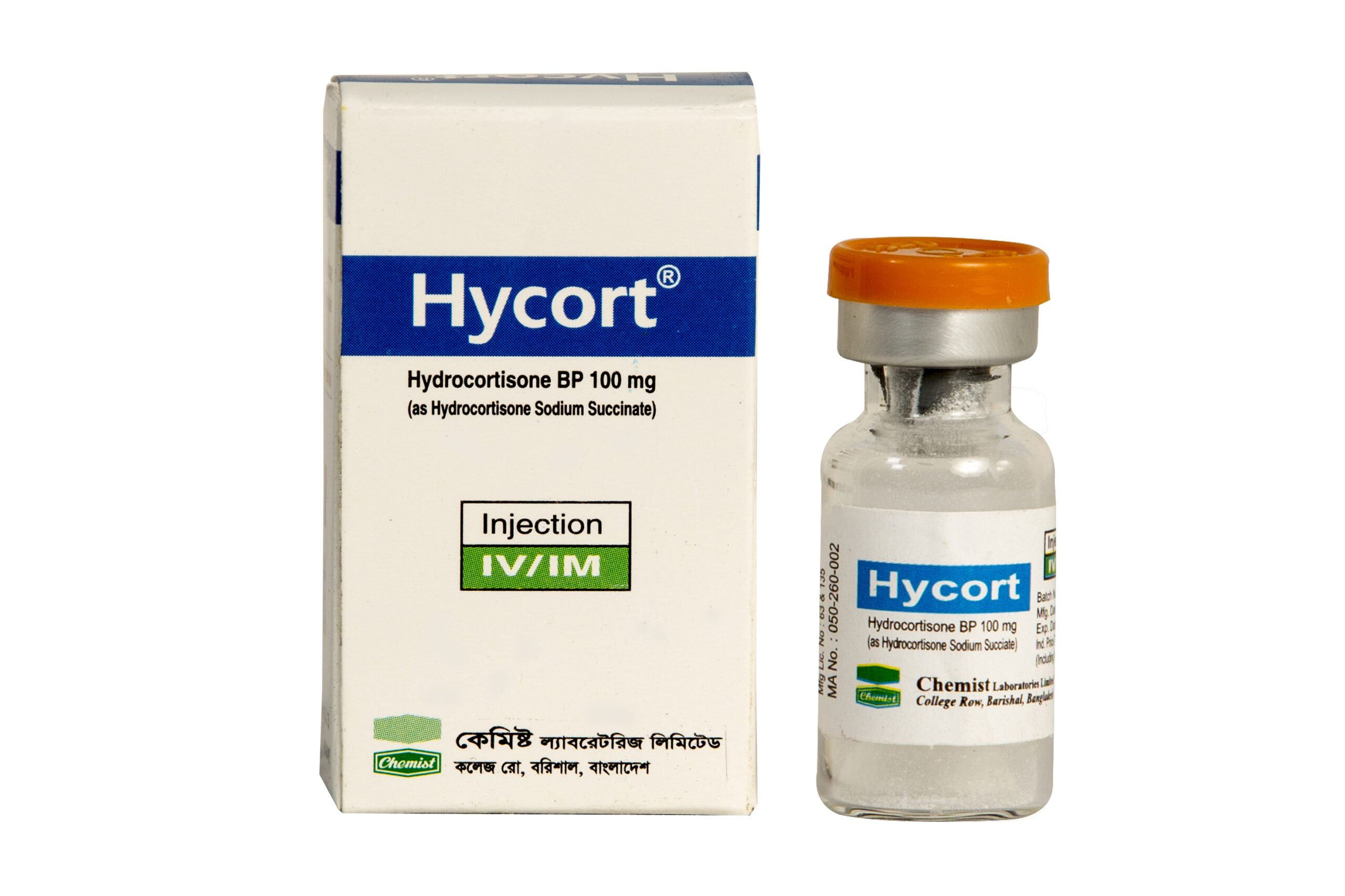
Hydrocortisone Sodium Succinate BP 100 mg/2 ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Hydrocortisone Sodium Succinate BP 100 mg/2 ml
Therapeutic Class: Glucocorticoids
Indication: Hycort is an injectable preparation of Hydrocortisone BP which is a synthetic corticosteroid. Hydrocortisone is indicated for use in the following conditions: Primary or secondary adrenocortical insufficiency Acute adrenocortical insufficiency Shock unresponsive to conventional therapy Congenital adrenal hyperplasia Hypercalcemia associated with cancer Non-suppurative thyroiditis Rheumatic Disorders Dermatologic Diseases (Allergic States, Severe seborrheic dermatitis, Severe psoriasis, Pemphigus, Severe erythema multiforme) Control of severe or incapacitating allergic conditions (Bronchial asthma, Contact dermatitis, Atopic dermatitis, Serum sickness, Seasonal or perennial allergic rhinitis, Drug hypersensitivity reactions, Urticarial transfusion reactions, Acute noninfectious laryngeal edema) Ophthalmic Diseases (Herpes zoster ophthalmicus, Iritis, iridocyclitis, Chorioretinitis, Diffuse posterior uveitis and choroiditis, Optic neuritis) Gastrointestinal Diseases Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy Loeffler's syndrome Aspiration pneumonitis Hematologic Disorders (Acquired, autoimmune hemolytic anemia, Idiopathic thrombocytopenic purpura in adults, Secondary thrombocytopenia, Erythroblastopenia) Neoplastic Diseases (Leukemias and lymphomas in adults, Acute leukemia of childhood) Edematous States Acute exacerbations of multiple sclerosis
Dosage & Administration: Adult : by IM injection or slow IV injection or infusion, 100-500 mg, 3-4 times in 24 hours or as required. Child: by slow IV Injection, up to 1 year: 25 mg, 1-5 years: 50 mg, 6-12 years: 100 mg.
Preparation: 1 ml X 1 Injection.
Methyl Prednisolone Acetate BP 80 mg/2 ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Methyl Prednisolone Acetate BP 80 mg/2 ml
Therapeutic Class: Glucocorticoids
Indication: Methylprednisolone is a prescription medication used to reduce inflammation. It has immune suppresive effect. Methylprednisolone Acetate is indicated in the treatment of asthma, atopic dermatitis, contact dermatitis, bullous dermatitis herpetiformis, mycosis fungoides, pemphigus, severe erythema multiforme, primary or secondary adrenocortical insufficiency, hypercalcemia associated with cancer, nonsuppurative thyroiditis, ulcerative colitis, hematologic disorders, palliative management of leukemias and lymphomas, sympathetic ophthalmia, keratitis, allergic conjunctivitis, Juvenile rheumatoid arthritis, ankylosing spondylitis, symptomatic sarcoidosis, aspiration pneumonitis.
Dosage & Administration: By intramuscular & intra-articular administration. For intra-articular injection : Rheumatoid and Osteoarthritis: The dose for intra-articular administration depends upon the size of joint and varies with the severity of the condition in the individual patients. Size of joint: Large- 20 to 80 mg, Medium- 10 to 40 mg, Small- 4 to 10 mg Adrenogenital syndrome : A single intramuscular injection of 40 mg every two weeks may be adequate. For maintenance of patients with rheumatoid arthritis, the weekly intramuscular dose will vary from 40 to 120 mg. The usual dosage for patients with dermatologic lesions benefited by systemic corticoid therapy is 40 to 120 mg of methylprednisolone acetate administered intramuscularly at weekly intervals for one to four weeks. In acute severe dermatitis due to poison ivy, relief may result within 8 to 12 hours following intramuscular administration of a single dose of 80 to 120 mg. In chronic contact dermatitis repeated injections at 5 to 0 day intervals may be necessary. In seborrheic dermatitis, a weekly dose of 80 mg may be adequate to control the condition. Following intramuscular administration of 80 to 120 mg asthmatic patients, relief may result within 6 to 48 hours and persist for several days to weeks. Similarly in patients with allergic rhinitis an intramuscular dose of 80 to 120 mg may be followed by relief of coryzal symptoms within six hours persisting for several days to three weeks. Multiple Sclerosis: In treatment of acute exacerbations of multiple sclerosis, daily doses of 160 mg of methylprednisolone for a week followed by 64mg every other day for 1 month have been shown to be effective (4mg of methylprednisolone is equivalent to 5mg of prednisolone). Or, as directed by the registered physician.
Preparation: 2 ml x2 Injection
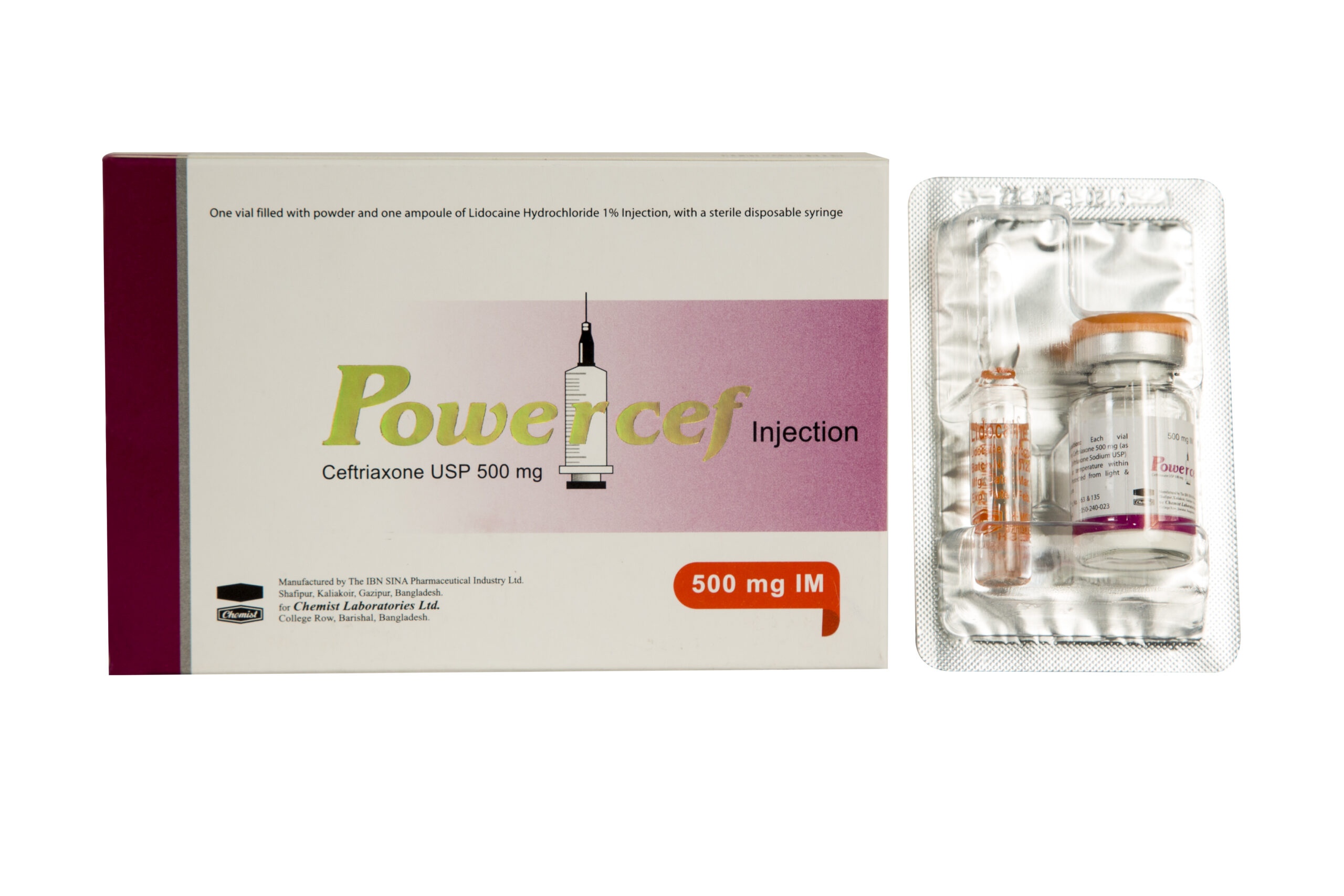
Ceftriaxone USP 500 mg IM
Business Unit: Human
Medicine Type: Injectable
Generic Name: Ceftriaxone USP 500 mg IM
Therapeutic Class: Third generation Cephalosporins
Indication: Powercef (Ceftriaxone USP) is a broad spectrum, sterile third generation bactericidal cephalosporin antibiotic for IM/IV administration. It is effective for Surgical Prophylaxis, Lower RTIs, Pelvic Inflammatory Disease, SSTI, UTI, Intra-Abdominal Infections, Meningitis, Bone & Joint Infections, Bacterial Septicemia, Uncomplicated Gonorrhea etc.
Dosage & Administration: Adult: The usual dose is 1 to 2 gm by intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Acute bacterial otitis media, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections, Meningitis: 1 to 2 g IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Uncomplicated gonococcal infections: 250 mg IM as a single dose. Surgical prophylaxis: 1 gm IV as a single dose 30 to 120 minutes before surgery. Infants and Children (01 month or older): The usual dose is 50 to 75 mg/kg intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections: 50 to 75 mg/kg IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 2 gm/day. Acute bacterial otitis media: 50 mg/kg IM in single dose; Maximum dose: 1 gm/day. Meningitis: 100 mg/kg IV or IM in single daily dose or (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Duration of therapy: Continue for more than 2 days after signs and symptoms of infection have disappeared. Usual duration is 4 to 14 days; in complicated infections, longer therapy may be required.
Preparation: 500 mg X 1 Injection.
Ceftriaxone USP 250 mg IM
Business Unit: Human
Medicine Type: Injectable
Generic Name: Ceftriaxone USP 250 mg IM
Therapeutic Class: Third generation Cephalosporins
Indication: Powercef (Ceftriaxone USP) is a broad spectrum, sterile third generation bactericidal cephalosporin antibiotic for IM/IV administration. It is effective for Surgical Prophylaxis, Lower RTIs, Pelvic Inflammatory Disease, SSTI, UTI, Intra-Abdominal Infections, Meningitis, Bone & Joint Infections, Bacterial Septicemia, Uncomplicated Gonorrhea etc.
Dosage & Administration: Adult: The usual dose is 1 to 2 gm by intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Acute bacterial otitis media, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections, Meningitis: 1 to 2 g IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Uncomplicated gonococcal infections: 250 mg IM as a single dose. Surgical prophylaxis: 1 gm IV as a single dose 30 to 120 minutes before surgery. Infants and Children (01 month or older): The usual dose is 50 to 75 mg/kg intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections: 50 to 75 mg/kg IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 2 gm/day. Acute bacterial otitis media: 50 mg/kg IM in single dose; Maximum dose: 1 gm/day. Meningitis: 100 mg/kg IV or IM in single daily dose or (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Duration of therapy: Continue for more than 2 days after signs and symptoms of infection have disappeared. Usual duration is 4 to 14 days; in complicated infections, longer therapy may be required.
Preparation: 250 mg X 1 Injection.
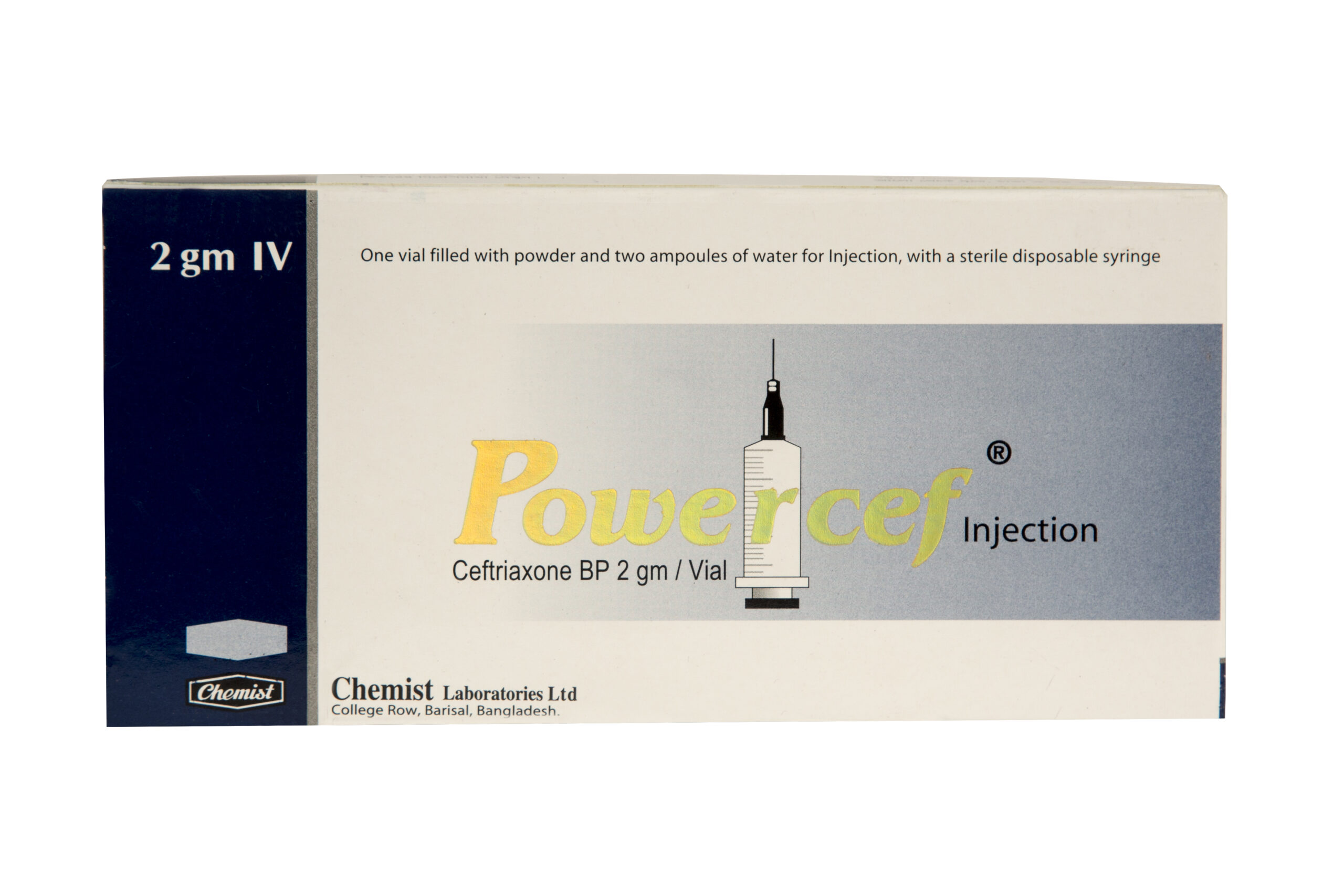
Ceftriaxone USP 2 gm IV
Business Unit: Human
Medicine Type: Injectable
Generic Name: Ceftriaxone USP 2 gm IV
Therapeutic Class: Third Generation Cephalosporins
Indication: Powercef (Ceftriaxone USP ) is effective for Surgical Prophylaxis, Lower RTIs, Pelvic Inflammatory Disease, SSTI, UTI, Intra-Abdominal Infections, Meningitis, Bone & Joint Infections, Bacterial Septicemia, Uncomplicated Gonorrhea etc.
Dosage & Administration: Adult: The usual dose is 1 to 2 gm by intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Acute bacterial otitis media, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections, Meningitis: 1 to 2 g IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Uncomplicated gonococcal infections: 250 mg IM as a single dose. Surgical prophylaxis: 1 gm IV as a single dose 30 to 120 minutes before surgery. Infants and Children (01 month or older): The usual dose is 50 to 75 mg/kg intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections: 50 to 75 mg/kg IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 2 gm/day. Acute bacterial otitis media: 50 mg/kg IM in single dose; Maximum dose: 1 gm/day. Meningitis: 100 mg/kg IV or IM in single daily dose or (or in equally divided doses twice a day); Maximum dose: 4 gm/day.
Preparation: 2 gm X 1 Injection.
Ceftriaxone USP 1gm IV
Business Unit: Human
Medicine Type: Injectable
Generic Name: Ceftriaxone USP 1gm IV
Therapeutic Class: Third generation Cephalosporins
Indication: Powercef (Ceftriaxone USP) is a broad spectrum, sterile third generation bactericidal cephalosporin antibiotic for IM/IV administration. It is effective for Surgical Prophylaxis, Lower RTIs, Pelvic Inflammatory Disease, SSTI, UTI, Intra-Abdominal Infections, Meningitis, Bone & Joint Infections, Bacterial Septicemia, Uncomplicated Gonorrhea etc.
Dosage & Administration: Adult: The usual dose is 1 to 2 gm by intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Acute bacterial otitis media, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections, Meningitis: 1 to 2 g IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Uncomplicated gonococcal infections: 250 mg IM as a single dose. Surgical prophylaxis: 1 gm IV as a single dose 30 to 120 minutes before surgery. Infants and Children (01 month or older): The usual dose is 50 to 75 mg/kg intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections: 50 to 75 mg/kg IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 2 gm/day. Acute bacterial otitis media: 50 mg/kg IM in single dose; Maximum dose: 1 gm/day. Meningitis: 100 mg/kg IV or IM in single daily dose or (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Duration of therapy: Continue for more than 2 days after signs and symptoms of infection have disappeared. Usual duration is 4 to 14 days; in complicated infections, longer therapy may be required.
Preparation: 1 gm X 1 Injection.
Ceftriaxone USP 1gm IM
Business Unit: Human
Medicine Type: Injectable
Generic Name: Ceftriaxone USP 1gm IM
Therapeutic Class: Third generation Cephalosporins
Indication: Powercef (Ceftriaxone USP) is a broad spectrum, sterile third generation bactericidal cephalosporin antibiotic for IM/IV administration. It is effective for Surgical Prophylaxis, Lower RTIs, Pelvic Inflammatory Disease, SSTI, UTI, Intra-Abdominal Infections, Meningitis, Bone & Joint Infections, Bacterial Septicemia, Uncomplicated Gonorrhea etc.
Dosage & Administration: Adult: The usual dose is 1 to 2 gm by intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Acute bacterial otitis media, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections, Meningitis: 1 to 2 g IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Uncomplicated gonococcal infections: 250 mg IM as a single dose. Surgical prophylaxis: 1 gm IV as a single dose 30 to 120 minutes before surgery. Infants and Children (01 month or older): The usual dose is 50 to 75 mg/kg intravenous or intramuscular administration once a day (or in equally divided doses twice a day). Pneumonia, Bronchitis, Skin and skin structure infection, Urinary tract infections, Bacterial Septicemia, Bone and joint infections: 50 to 75 mg/kg IV or IM once a day (or in equally divided doses twice a day); Maximum dose: 2 gm/day. Acute bacterial otitis media: 50 mg/kg IM in single dose; Maximum dose: 1 gm/day. Meningitis: 100 mg/kg IV or IM in single daily dose or (or in equally divided doses twice a day); Maximum dose: 4 gm/day. Duration of therapy: Continue for more than 2 days after signs and symptoms of infection have disappeared. Usual duration is 4 to 14 days; in complicated infections, longer therapy may be required.
Preparation: 1 gm X 1 Injection.
Colecalciferol [Vit-D3] 200000 IU/ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Colecalciferol [Vit-D3] 200000 IU/ml
Therapeutic Class: Vitamin-D preparations, Vitamin in bone formation.
Indication: Debolin injection is a parenteral preparation of Colecalciferol (Vitamin D3). Vitamin D3 is essential for normal bone growth and development and to maintain bone density. It is also necessary for utilization of both Calcium and Phosphorus. It is indicated for treatment of Osteoporosis, Hypoparathyroidism, Osteomalacia, Rickets, Vitamin D deficiency.
Dosage & Administration: Prevention: Infants receiving Vitamin D enriched milk: 1/2 ampoule (0.5ml) i.e. 1,00000 IU. every 6 months. Nursed infants or infants not receiving Vitamin D enriched milk or young children up to 5 years of age: 1 ampoule (1ml) i.e. 2,00000 I.U. every 6 months. Adolescents: 1 ampoule (1ml) i.e. 2,00000 IU every 6 months during winter. Pregnancy: 1/2 ampoule (0.5ml) i.e. 1,00000 IU from the 6th or 7th month of pregnancy. Elderly: 1/2 ampoule (0.5ml) i.e. 1,00000 IU every 3 months. Digestive disorders, concomitant treatment with antiepileptics & other particular condition not described above; 1/2 ampoule (0.5ml) i.e. 1,00000 IU or 1 ampoule (1ml) i.e. 2,00000 IU every 3 or 6 months. Vitamin D deficiency: 1 ampoule (1ml) i.e. 2,00000 IU which can be repeated 1 to 6 months later. Or, as directed by the registered physician.
Preparation: 1 ml X 5 Injections.
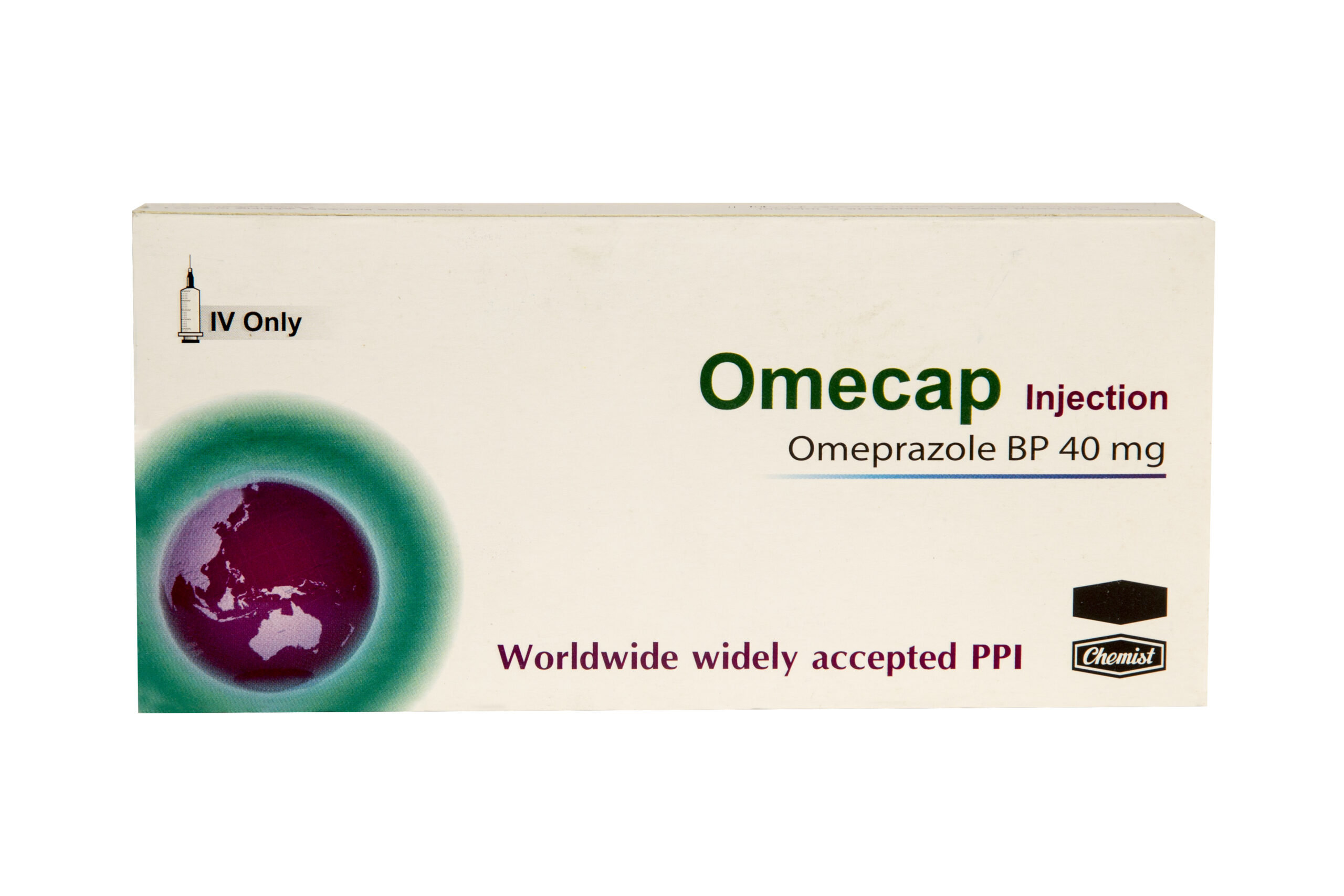
Omeprazole BP 40 mg/vial
Business Unit: Human
Medicine Type: Injectable
Generic Name: Omeprazole BP 40 mg/vial
Therapeutic Class: Anti-ulcerant - Proton Pump Inhibitor
Indication: Omecap is an ideal preparation of Omeprazole which is a proton pump inhibitor. Omeprazole is indicated for the treatment of- Gastric and duodenal ulcer NSAID-associated duodenal and gastric ulcer As prophylaxis in patients with a history of NSAID-associated duodenal and gastric ulcer Gastro-esophageal reflux disease Long-term management of acid reflux disease Acid-related dyspepsia Severe ulcerating reflux esophagitis Prophylaxis of acid aspiration during general anesthesia Zollinger-Ellison syndrome Helicobacter pylori-induced peptic ulcer.
Dosage & Administration: IV Injection- Prophylaxis of acid aspiration: Omeprazole 40 mg to be given slowly (over a period of 5 minutes) as an intravenous injection, one hour before surgery. Duodenal ulcer, gastric ulcer or reflux oesophagitis: In patients with duodenal ulcer, gastric ulcer or reflux oesophagitis where oral medication is inappropriate, Omeprazole IV 40 mg once daily is recommended. Zollinger- Ellison syndrome (ZES): In patients with Zollinger-Ellison Syndrome the recommended initial dose of Omeprazole given intravenously is 60 mg daily. Higher daily doses may be required and the dose should be adjusted individually. When doses exceed 60 mg daily, the dose should be divided & given twice daily.
Preparation: 1 ml x1 Injection
Diclofenac Sodium BP + Lidocaine Hydrochloride USP (75 mg + 20 mg) / 2 ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Diclofenac Sodium BP + Lidocaine Hydrochloride USP (75 mg + 20 mg) / 2 ml
Therapeutic Class: Non-steroidal Anti-inflammatory Drugs (NSAIDs), Drugs used for Rheumatoid Arthritis, Drugs for Osteoarthritis.
Indication: C-Fenac Plus is an Injection preparation of Diclofenac Sodium with Lidocaine Hydrochloride. Diclofenac is a potent Non-Steroidal Anti-Inflammatory Drug (NSAID) with marked analgesic and antipyretic properties. Lidocaine acts more rapidly and is more stable than most other local anaesthetics. Lidocaine impairs the generation and conduction of the nerve impulses by slowing depolarization. Indicated for Rheumatoid arthritis, Osteoarthritis, Ankylosing spondylitis, Pain, Acute gout, Inflammation, Tendinitis, Actinic keratosis, Bursitis.
Dosage & Administration: Each 2 ml ampoule contains Diclofenac Sodium 75 mg and Lidocaine Hydrochloride 20 mg. Adults: One ampoule once (or in severe cases, twice) daily by intramuscular injection. Renal colic: One ampoule once daily intramuscularly. A further ampoule may be administered after 30 minutes, if necessary. The recommended maximum daily dose of diclofenac is 150 mg, by any route. The recommended maximum daily dose of lidocaine is 200 mg. Children: In juvenile chronic arthritis, 1-3 mg of diclofenac/kg body wt. daily in divided doses. Elderly patients: In elderly or debilitated patients, the lowest effective dosage is recommended, commensurate with age and physical status.
Preparation: 2 ml x 20 Injection
Diclofenac Sodium BP 75 mg / 3 ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Diclofenac Sodium BP 75 mg / 3 ml
Therapeutic Class: Non-steroidal Anti-inflammatory Drugs (NSAIDs), Drugs used for Rheumatoid Arthritis, Drugs for Osteoarthritis.
Indication: C-Fenac is a preparation of Diclofenac Sodium. Diclofenac is a potent Non-Steroidal Anti-Inflammatory Drug (NSAID) with marked analgesic and antipyretic properties. Diclofenac inhibits the synthesis of prostaglandins by inhibiting cycloxygenase enzyme. C-Fenac is indicated for Rheumatoid arthritis, Osteoarthritis, Ankylosing spondylitis, Pain, Acute gout, Inflammation, Tendinitis, Actinic keratosis, Bursitis.
Dosage & Administration: Adult: One ampoule once (or in severe cases, twice) daily by intramuscular injection
Preparation: 3 ml x 10 Injection
Vitamin K1 (Phytomenadione) 2 mg/0.2 ml.
Business Unit: Human
Medicine Type: Injectable
Generic Name: Vitamin K1 (Phytomenadione) 2 mg/0.2 ml.
Therapeutic Class: Vitamin-K Preparations
Indication: Babykion is a preparation of Phytomenadion (Vitamin K1). Vitamin K1 (Phytomenadione) is a procoagulant factor. Phytomenadione (Vitamin K-1) is indicated in following indications- Prophylaxis and treatment of haemorrhagic disease in the newborn. Haemorrhage or risk of haemorrhage as a result of severe hypoprothrombinemia (i.e. deficiency of clotting factors II, VII, IX and X) of various etiologies, including overdosage of courmarin-type anticoagulants, their combination with phenylbutazone, and other forms of hypovitaminosis K (e.g. in obstructive jaundice as well as liver and intestinal disorders, and after prolonged treatment with antibiotics, sulphonamides or salicylates). Prevention and treatment of bleeding due to vitamin K deficiency.
Dosage & Administration: 1st Dose: Within four hours of birth orally. 2nd Dose : At the age of four days of birth orally. 3rd Dose : At the age of four weeks of birth orally. Prophylaxis: Mild Hemorrhage or hemorrhagic tendency: The usual dose for Neonates is 2 mg orally at or just after birth. Then 2 mg on 4th-5th day and another 2 mg on 28th-30th day orally. If the oral route is unsuitable then 2 mg of drug can be administered by IM or IV route. Children over 1 year of age: Could be given 5-10 mg orally. A single 1 mg (0.1 ml) dose IM is recommended in children who are not assured of receiving a second oral dose or, in the case of breast-fed children, who are not assured of receiving a third oral dose. Therapy: Initially, 1 mg by intravenous injection, with further doses as required, based on the clinical picture and coagulation status. Neonates with special risk factors: Pre-maturity, birth asphyxia (inadequate intake of oxygen by the baby during birth process), obstructive jaundice, inability to swallow, maternal use of anticoagulants or anti-epileptics- 1 mg intramuscularly or intravenously at birth or shortly after birth if the oral route is unsuitable. Intramuscular and intravenous doses should not exceed 0.4 mg/kg in premature infants weighing less than 2.5 kg. The size and frequency of further doses should be based on coagulation status To ensure a total protection of the newborns, 3 prophylactic doses of Vitamin K should be administered orally following the dosing schedule mentioned above.
Preparation: 0.2 ml x 5 Injection
Thiamine HCI
Business Unit: Human
Medicine Type: Injectable
Generic Name: Thiamine HCI
Therapeutic Class: Vitamina B praparation
Indication: Vitamin B1 is a preparation of Thiamine HCl. Thiamine, in the form of thiamine pyrophosphate, is the coenzyme for decarboxylation of α-ketoglutaric acid. Thiamine is well absorbed from the gastrointestinal tract and widely distributed throughout the body. This vitamin is necessary for the optimal growth of infants and children. Vitamin-B1 is specifically used in the treatment of the various manifestations of thiamine deficiency such as Beriberi and Wernick's encephalopathy, neuritis associated with pregnancy and pellagra.
Dosage & Administration: In the treatment of beriberi, 10 mg to 20 mg of thiamine hydrochloride are given IM 3 times daily for as long as 2 weeks.
Preparation: 1 ml x 50 Injection
Ranitidine HCI 150mg tablet & 50mg/2ml injection
Business Unit: Human
Medicine Type: Injectable Tablet
Generic Name: Ranitidine HCI 150mg tablet & 50mg/2ml injection
Therapeutic Class: Histamine receptor blocker
Indication: Ranix is formulation of Ranitidine which is known as H2 receptor antagonist. Ranix is indicated for Peptic Ulcer Disease (PUD), Gastroesophageal Reflux Disease (GERD), Zollinger-Ellison syndrome, Upper gastrointestinal bleeding and Stress ulcers.
Dosage & Administration: Oral: 150 mg 2 times a day, or 300 mg once a day after the evening meal . or at bed time. Parenteral: 50 mg, IV or IM, every 6 to 8 hours for up to 8 weeks
Preparation: 2ml x 10 Injection
Cyanocobalamin (Vit. B12) BP 1000 mcg
Business Unit: Human
Medicine Type: Injectable
Generic Name: Cyanocobalamin (Vit. B12) BP 1000 mcg
Therapeutic Class: Vitamin-B preparations, Drugs for Megaloblastic Anemia
Indication: Cynovit is a preparation of cyanocobalamin (B12). 1. It is used for maintaining normal vitamin B12 blood levels in certain patients with pernicious anemia. 2. It is also used to treat or prevent low blood levels of vitamin B12 due to low intake from food, 3. Thyrotoxicosis, 4. Hemorrhage, 5. Malignancy, 6. Liver or kidney disease, 7. Gastric bypass surgery, 8. Total or partial gastrectomy, Gluten enteropathy or sprue, 9. Folic acid deficiency, Macrocytic anaemia. Pregnancy Category : A.
Dosage & Administration: Initially : 1mg (1 ampoule) intramuscularly or deep subcutaneous once a day for 6 to 7 days. Maintenance : 1 mg (1 ampoule) every month.
Preparation: 1ml x 25 Injection
Tiemonium Methylsulfate 5mg/2ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Tiemonium Methylsulfate 5mg/2ml
Therapeutic Class: Anticholinergics - antispasmodic
Indication: Tienum is a preparation of Tiemonium Methylsulphate. Tiemonium Methylsulphate is an antispasmodic drug that reduces muscles spasm of the intestine, biliary system, bladder and uterus. Tienum is used in the symptomatic treatment of pain related to functional disorders of the digestive tract and biliary system. It is also indicated for the treatment of spasm and pain in urological and gynecological diseases.
Dosage & Administration: 1 ampoule (5mg) by slow IV/IM injection 3times daily.
Preparation: Each box contains 5 ampoules of 2 ml.
Aminocaproic Acid 200 mg/ml
Business Unit: Animal Health, Dairy
Medicine Type: Injectable
Generic Name: Aminocaproic Acid 200 mg/ml
Indication: "Cattle,Buffalo,Goat,Sheep : Reduce bleeding during surgery, any type of wound, bleeding due to injury. "
Dosage & Administration: "Cow, buffalo, camel, horse, goat,sheep: 10 ml/ 70-80 kg by IV or Orally."
Preparation: 10 ml
Dexamethasone Sodium Phosphate 5mg/ml
Business Unit: Human
Medicine Type: Injectable
Generic Name: Dexamethasone Sodium Phosphate 5mg/ml
Therapeutic Class: Glucocorticoids, Corticosteroid
Indication: 1. Allergic states: Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity reactions, perennial or seasonal allergic rhinitis and serum sickness. 2. Collagen disease: Like lupus erythematosus, rheumatoid arthritis etc. 3. Dermatologic diseases: Bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus and severe erythema multiforme (Stevens-Johnson syndrome). Endocrine disorders: Primary or secondary adrenocortical insufficiency, congenital adrenal hyperplasia, hypercalcemia associated with cancer and nonsuppurative thyroiditis. 4. Gastrointestinal diseases: Regional enteritis and ulcerative colitis. 5. Hematologic disorders: Acquired (autoimmune) hemolytic anemia, congenital (erythroid) hypoplastic anemia (Diamond-Blackfan anemia), idiopathic thrombocytopenic purpura in adults and selected cases of secondary thrombocytopenia. 6. Neoplastic diseases: Leukemias and lymphomas. 7. Nervous system: Acute exacerbations of multiple sclerosis, cerebral edema associated with primary or metastatic brain tumor, craniotomy or head injury. 8. Ophthalmic diseases: Temporal arteritis, uveitis, and ocular inflammatory conditions unresponsive to topical corticosteroids. 9. Renal diseases: To induce a diuresis or remission of proteinuria in idiopathic nephrotic syndrome or that due to lupus erythematosus. 10. Respiratory diseases: Berylliosis, fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis. 11. Rheumatic disorders: As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis, acute rheumatic carditis, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy). For the treatment of dermatomyositis, polymyositis, and systemic lupus erythematosus. 12. Miscellaneous: Diagnostic testing of adrenocortical hyperfunction, trichinosis with neurologic or myocardial involvement, tuberculous meningitis with subarachnoid block or impending block when used with appropriate antituberculous chemotherapy.
Dosage & Administration: In adult patients, daily oral dosages vary from 1 mg to 10 mg and in children from 0.03-0.20 mg/kg body weight,according to the individual response. 1. In some patients higher dosages may be temporarily required,to control the disease. As soon as circumstances permit,dosage should be decreased. 2. For a short dexamethasone suppression test 1 mg dexamethasone is given at 11 p.m. and plasmacortisol measured the next morning. 3. Patients who do not show a decrease in cortisol can be exposed to a longer test: 0.5 mg dexamethasone is given at 6-hour intervals. 4. For 48 hours followed by 2 mg every 6 hours for a further 48 hours. 24-hour urine collections are made before, during and at the end of the test for the determination of 17 alpha-hydroxycorticoids. For injection: Dexamethasone can be given by intravenous (IV), intramuscular (IM) or local injection. Dexamethasone injections can also be diluted with an infusion fluid or be injected directly into the infusion line. Intravenous injections of massive doses should be given slowly, over a period of several minutes. Intramuscular administration should be given by deep intramuscular injection,to prevent atrophy of the subcutaneous adipose tissues. Intra-articular injections should be given under strictly aseptic conditions as glucocorticoids decrease the resistance to infection. When diluted with these infusion fluids, Dexamethasone will keep its potency for at least 24 hours (at room temperature and in daylight conditions). As infusion fluids, Sodium chloride 0.9%, Anhydrous glucose 5%, Invert sugar 10%, Sorbitol 5%, Ringer's solution, Hartman's solution (Ringer-lactate) etc.can be used. The dosage of Dexamethasone depends on the severity of the condition and the response of the patient. For systemic therapy in adults, daily doses of 0.05-0.20 mg/kg body weight are usually sufficient. For emergencies (e.g. anaphylaxis, acute severe asthma, cerebral edema) substantially higher doses are required. An initial dose of 10-20 mg IV is followed by 6 mg IV or IM every 6 hours,until a satisfactory result has been obtained. Thereafter the dosage has to be tapered off gradually. For local therapy, the following doses are recommended: Intra-articularly: 2-4 mg in large and 0.8-1 mg in small joints Intrabursally: 2-4 mg;in tendon sheaths:0.4-1 mg The frequency of these injections may vary from every 3-5 days to every 2-3 weeks
Preparation: 2x10 ampoules of 1ml
Ketorolac Tromethamine USP 30mg/ml
Business Unit:
Human
Medicine Type: Injectable
Generic Name: Ketorolac Tromethamine USP 30mg/ml
Therapeutic Class: NSAIDs / Non Opioid Analgesic
Indication: Ketorolac Tromethamine is a potent analgesic of the non-steroidal anti-inflammatory drugs (NSAIDs). It acts by inhibiting the cyclooxygenase enzyme system and hence inhibits the prostaglandin synthesis. It demonstrates a minimal anti-inflammatory effect at its analgesic dose. Short-term management of moderate to severe acute post-operative pain and acute pain of other origins.
Dosage & Administration: Ketorolac injection may be used as a single or multiple doses, on a regular or when necessary schedule for the management of moderately severe, acute pain that requires analgesia at the opioid level, usually in a postoperative setting. When administering Ketorolac injection, the IV bolus must be given over no less than 15 seconds. The IM administration should be given slowly and deeply into the muscle. The analgesic effect begins within 30 minutes with maximum effect in 1 to 2 hours after dosing IV or IM. Duration of analgesic effect is usually 4 to 6 hours. Single-Dose Treatment- IM Dosing (Adult): Patients <65 years of age: One dose of 60 mg. Patients >65 years of age, renally impaired and/or less than 50 kg of body weight: One dose of 30 mg. IV Dosing (Adult): Patients <65 years of age: One dose of 30 mg. Patients >65 years of age, renally impaired and/or less than 50 kg of body weight: One dose of 15 mg. IV or IM Dosing (2 to 16 years of age): IM Dosing: One dose of 1 mg/kg up to a maximum of 30 mg. IV Dosing: One dose of 0.5 mg/kg up to a maximum of 15 mg. Multiple-Dose Treatment (IV or IM)- Patients <65 years of age: The recommended dose is 30 mg Ketorolac injection every 6 hours. The maximum daily dose should not exceed 120 mg. Patients >65 years of age, renally impaired patients and patients less than 50 kg: The recommended dose is 15 mg Ketorolac injection every 6 hours. The maximum daily dose for these populations should not exceed 60 mg. For breakthrough pain, do not increase the dose or the frequency of Ketorolac Tromethamine. Conversion from Parenteral to Oral Therapy: Ketorolac tablets may be used either as monotherapy or as follow-on therapy to parenteral Ketorolac. When Ketorolac tablets are used as a follow-on therapy to parenteral Ketorolac, the total combined daily dose of ketorolac (oral + parenteral) should not exceed 120 mg in younger adult patients or 60 mg in elderly patients on the day the change of formulation is made. On subsequent days, oral dosing should not exceed the recommended daily maximum of 40 mg. Ketorolac IM should be replaced by Ketorolac tablet as soon as feasible. The total duration of combined parenteral and oral treatment should not exceed 5 days.
Preparation: Kelac Injection: 30ml/1ml x 6